|
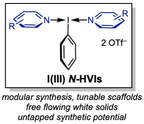
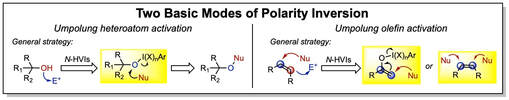
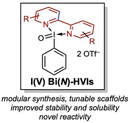
Our lab aims to develop a multifaceted research program that leverages a unique class of nitrogen-ligated hypervalent iodine reagents, N-HVIs, in novel approaches to "umpolung" or reverse-polarity chemistry, At present, our projects can be categorized into the inversion of two main classes of functional groups, "umpoled heteroatoms" and "umpoled olefins". Our research efforts encompasses reagent development, new synthetic methods, and the application of these tools to the synthesis of natural products with a special interest in those containing challenging medium-ring heterocycles. We aim to bridge the gap between the three-dimensional molecular complexity of nature and the planar aromatic structures of small molecule libraries by introducing simplified strategies and novel disconnections of these valuable architectures.
|
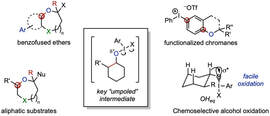
Umpoled Heteroatom MethodologiesBy rendering heteroatoms including oxygen, nitrogen, and sulfur electrophilic through ligation to an I(III) reagent, we devise new ways to leverage these ubiquitous functional handles in organic synthesis. This includes the synthesis of medium-sized rings via oxidative ring expansions, arene C–H functionalization, and new selective oxidations.
|
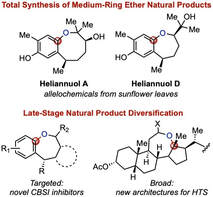
Medium-Rings in Natural Product SynthesisWe have an interest in natural products containing medium-sized rings, both heterocyclic and carbocyclic. We use these scaffolds as opportunities for novel reaction development and the development of concise, scalable approaches to these molecules. We are also interested in studying the biology of complex medium-ring natural product analogues, accessed via late-stage diversifications.
|
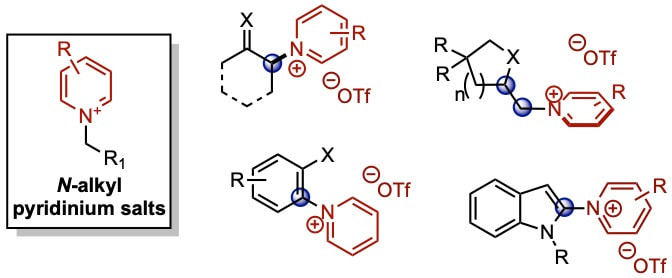
Pyrdinium Salt Synthesis via
|
FUNDING
The Wengryniuk Lab is grateful to the following funding agencies for their support of our work!