|
The W Lab, Summer 2023
|
The W Lab @ TempleSustainable Approaches to Reversed-Polarity TransformationsIn the W lab, our diverse team of researchers work on a multifaceted research program that focused on sustainable approaches to "umpolung" or reverse-polarity chemistry. Our research encompasses reagent development, new synthetic methods, catalysis, and the application of these tools to the synthesis of natural products. We are particularly interested in methods that allow rapid access to molecular diversity, either via modular small molecule library synthesis or late-stage natural product derivatization.
|
W Lab News
Summer 2024: Congratulations to our undergraduate Zach Christidhis for graduating with his B.S. in chemistry. He will be pursuing his graduate studies at Johns Hopkins in the fall!
Summer 2024: Congratulations to Bill Motsch for successfully defending his thesis and starting his post-doc at GSK!
Temple O.W.L.S
Are you woman in STEM at Temple? Looking for resources, networking and outreach opportunities?
Visit our website to learn more about the Temple O.W.L.S.
Incoming female first years: Be sure to check out our O.W.L.S. Mentorship Program!
Visit our website to learn more about the Temple O.W.L.S.
Incoming female first years: Be sure to check out our O.W.L.S. Mentorship Program!
Recent Research Highlights
|
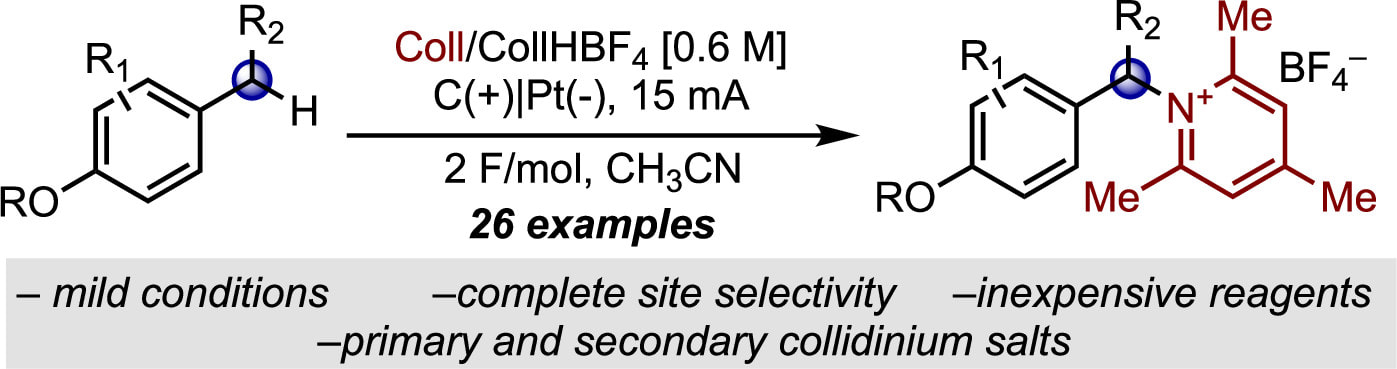
An electrifying approach to sterically-hindered pyridinium salts! Our recent OL (Org. Lett. 2022, 24, 6060.) outlines our method for the site-selective synthesis of benzylic 2,4,6-collidinium salts via electrooxidative C–H functionalization. 2,4,6-Collidinium salts are emerging as a cost and atom-economical alternative to Katritzky salts for cross couplings, and now we can make them directly from C–H bonds! We hope this chromatography free method will get people excited about the growing potential that N-alkyl pyridinium salts have as versatile redox-active functional handles.
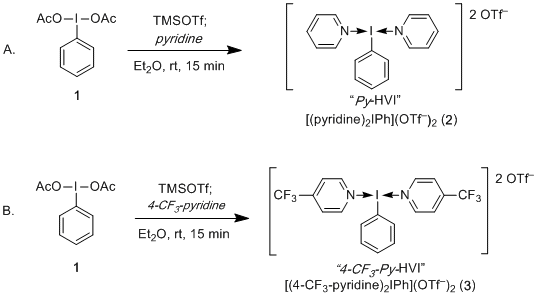
Need to know how to make these mysterious N-HVIs? Look no further! Bilal has compiled the ultimate guide in our Organic Syntheses report (Org. Syn. 2021, 98, 391. We detail the synthesis of both Py-HVI and 4-CF3-Py-HVI, representative of the variable N-HVI stability. Included are three isolation procedures, including bench top, vacuum, and glove box filtration. If the N-HVI can be made, one of these techniques is how you do it.
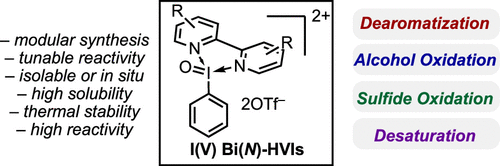
Still want more insights? Feel free to shoot us an email! I(V) Bi(N)-HVIs: They do more than dearomatize! Our J. Org. Chem. report (JOC, 2021, 86, 6566) profiles the synthesis, reactivity, and structure (thanks Ariafard group!) of a library of Bi(N)-HVI reagents. They are highly efficient oxidants in dearomatization, alcohol and sulfide oxidation, and ketone desaturation. One compelling practical feature of these scaffolds is their improved solubility and stability relative to common I(V) reagents. Additionally, their in situ application makes for an efficient approach to their use from simply PhIO2.
Check 'em out for your next oxidation challenge! |